Nanotechnology to unravel crystallisation mechanisms
May 25, 2023
Crystallisation is the process by which atoms or molecules arrange into a highly organised rigid structure, known as a crystal. One can observe crystallisation in nature, such as when water turns into snow, or during the mineral formation of magma after a volcanic eruption.
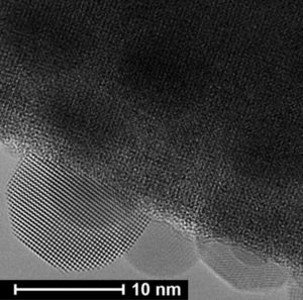
This figure shows the formation of the Bismuth crystal. The electron beam irradiation, using in situ electron microscopy, induces the formation of the liquid droplet and its transformation to the crystal. Electron microscope image obtained by Junjie Li.
Crystallisation is the basis for the formation of several materials including metals, ice, colloids and proteins, and it has several industrial applications, including in pharmaceutical, chemical or food industries. The mystery of crystallisation lies in how crystals with long-range ordered structures originate from the fundamental building blocks like ions, atoms, or molecules, which only interact with their neighbours.
The early stages happening during crystallisation play a critical role in defining the properties of the crystal, such as the crystal structure, its shape, size distribution and purity. Understanding the crystallisation mechanisms, namely nucleation and growth, is of great interest to scientists in many different disciplines. However, direct atomic-scale observation is still a significant challenge. INL researchers use advanced electron microscopic techniques in combination with image processing, simulations and theoretical calculations to unravel crystallisation steps at the atomic scale.
The Nanostructured Materials research group employs in situ electron microscopy to investigate the dynamics of nucleation and growth at the atomic scale, to discover and uncover non-classical mechanisms. Leonard Francis explains that “the studies provide important insights about the formation of the initial crystal nucleus, helping to answer relevant questions about the stability of nano-sized clusters and what is the smallest nanocrystal size, clarify crystal growth mechanisms as well as advance the general understanding of the dynamic process of nucleation and growth of materials and phase transformations at the atomic scale.”