Revolutionising cancer monitoring: INL’s microfluidic chip enables earlier diagnosis and personalised treatment
September 14, 2023
Circulating tumour cells, or CTCs, are the agents responsible for cancer’s spread to distant parts of the body and have been an enigma in the realm of cancer research. Their analysis, termed liquid biopsy, allows for non-invasive and continuous monitoring of cancer through a simple blood test. However, until now, this analysis has been limited by the technologies available for CTC isolation which were primarily dependent on immuno-recognition and only captured a subgroup of CTCs (typically the less aggressive type).
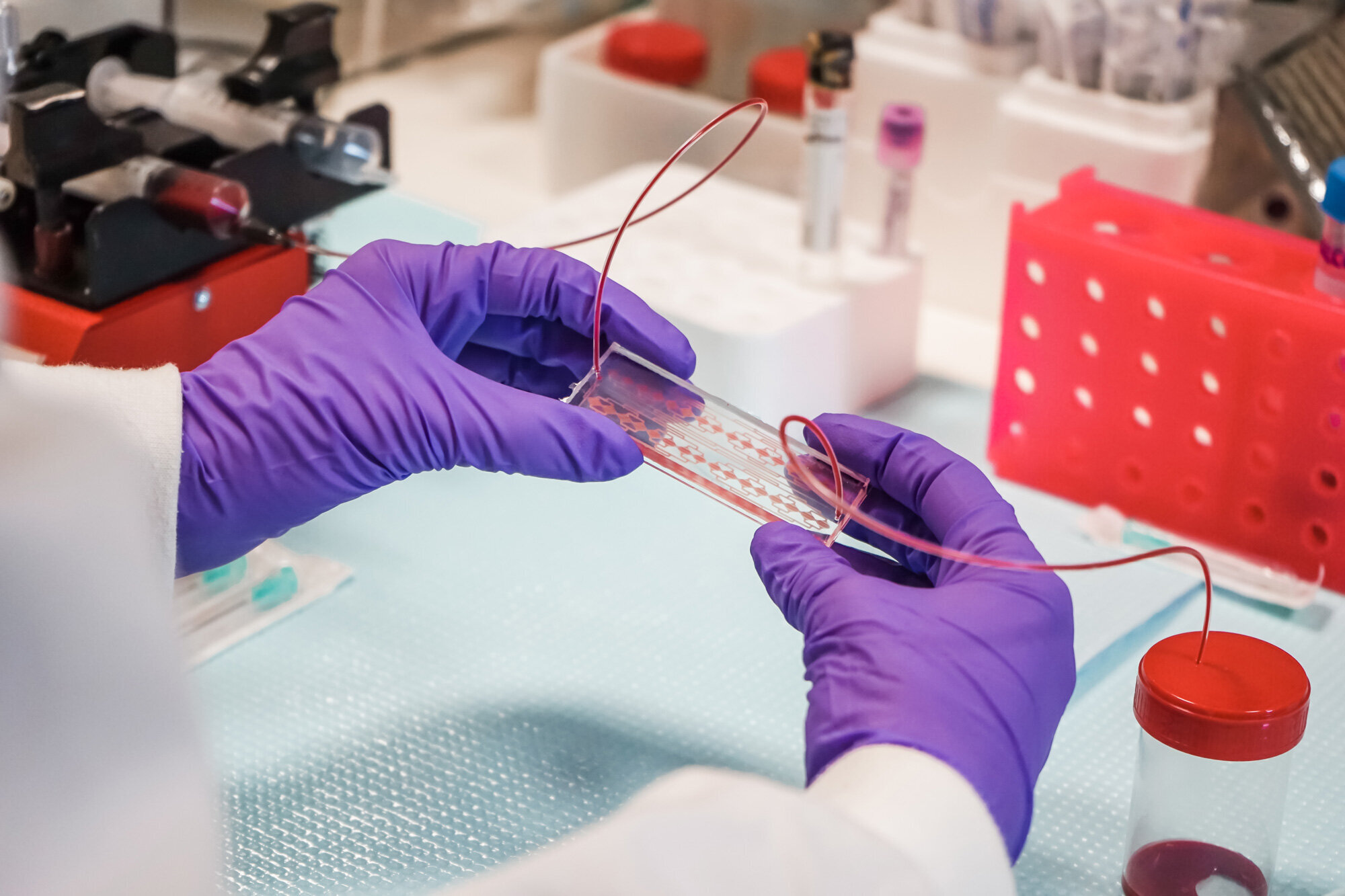
The Medical Devices research group at INL has pioneered a microfluidic system that holds the potential to transform the way we understand and fight cancer. This pioneering microfluidic chip marks a paradigm shift.
The revolutionary system can efficiently and swiftly isolate all types of CTCs directly from whole blood samples, eliminating the need for complex pre-processing steps. The microfluidic chip also facilitates high-resolution imaging of the CTCs, enabling automation of the entire processing and analysis method, thus reducing user dependence, and has already been demonstrated in clinical testing to predict cancer progression up to 1 year earlier than existing technologies.
The project CTC-OncoDynamics funded through the Caixa Impulse Consolidate program, and done in collaboration with INL’s spin-off company RUBYnanomed, aimed at increasing the maturity of this technology and to demonstrate its performance on a cohort of metastatic breast cancer patients. This critical step seeks to evaluate the prognostic value of CTCs and empower oncologists with a powerful tool that offers insights into disease progression and that aids the selection of personalised therapy strategies, ensuring that patients receive treatments tailored to their unique needs.
During the project that was recently finished in Summer 2023, researchers had the opportunity to demonstrate the technology in metastatic breast cancer and gastrointestinal and genitourinary tumours. The results of this clinical testing have highlighted the advantages of this technology. Lorena Diéguez, the principal investigator of CTC-OncoDynamics and Medical Devices group leader, explains that “the enumeration and phenotypic analysis of the isolated CTCs enabled the classification of patients in different risk groups. Moreover, the chip’s ability to select suitable patient populations for targeted therapies exemplifies its potential in revolutionising personalised medicine.”
From enabling more frequent non-invasive monitoring, to facilitating earlier and more precise diagnoses, and ultimately leading to successful personalised medicine, this technological innovation also holds the potential to significantly reduce public healthcare costs by an estimated 40%.
This innovation has been transferred to RUBYnanomed which pursues the certification and commercialisation of the technology, supported by the first EIC accelerator in Portugal, which started in August 2022.